Pure substance vs mixture worksheet – Embark on a journey into the realm of chemistry with our captivating pure substance vs. mixture worksheet. This comprehensive resource delves into the fundamental concepts that distinguish these two entities, providing a solid foundation for your understanding of matter.
As we explore the unique properties and applications of pure substances and mixtures, you’ll gain valuable insights into their significance in both the scientific and everyday world.
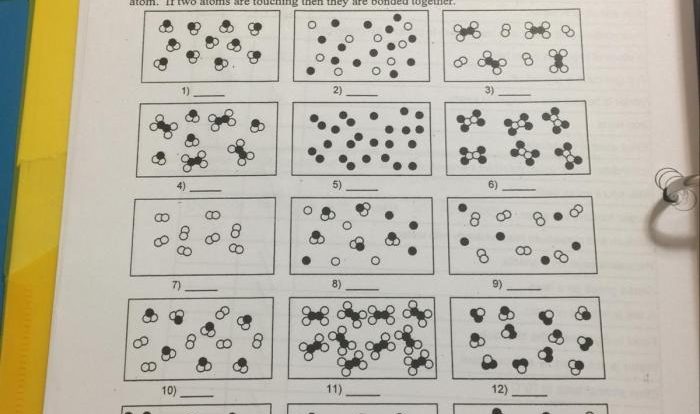
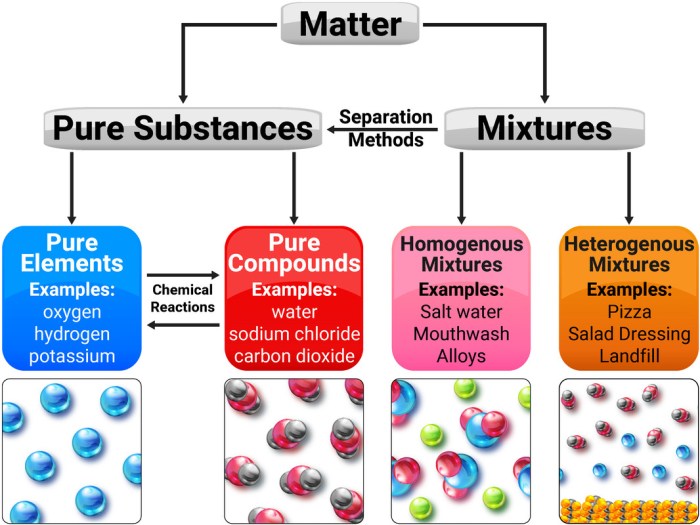
1. Pure Substance vs. Mixture Definitions
A pure substance is a substance that has a constant composition and distinct properties. It cannot be broken down into simpler substances by chemical means. Examples of pure substances include elements, such as gold and oxygen, and compounds, such as water and carbon dioxide.
A mixture is a combination of two or more pure substances that are not chemically bonded. The composition of a mixture can vary, and the properties of a mixture can be different from the properties of its individual components. Examples of mixtures include salt water, air, and gasoline.
2. Properties of Pure Substances and Mixtures
Characteristic Properties of Pure Substances
Pure substances have characteristic properties that can be used to identify them. These properties include:
- Melting point
- Boiling point
- Density
- Refractive index
Properties of Mixtures
The properties of mixtures can vary depending on the composition of the mixture. However, some general properties of mixtures include:
- The composition of a mixture can vary.
- The properties of a mixture can be different from the properties of its individual components.
- Mixtures can be separated into their individual components by physical means, such as filtration, distillation, and chromatography.
3. Separation of Pure Substances from Mixtures
There are a variety of methods that can be used to separate pure substances from mixtures. These methods include:
- Filtration
- Distillation
- Chromatography
The choice of separation method depends on the nature of the mixture and the desired purity of the separated substances.
4. Applications of Pure Substances and Mixtures
Applications of Pure Substances
Pure substances have a wide range of applications in science and industry. Some of the most important applications include:
- As reagents in chemical reactions
- As solvents
- As catalysts
- As semiconductors
Applications of Mixtures, Pure substance vs mixture worksheet
Mixtures are used in a wide variety of everyday applications. Some of the most common applications include:
- As food
- As beverages
- As fuels
- As cleaning agents
FAQ Insights: Pure Substance Vs Mixture Worksheet
What is the key difference between a pure substance and a mixture?
A pure substance is a substance with a uniform composition and distinct properties, while a mixture is a combination of two or more substances that retain their individual identities.
How can we separate a pure substance from a mixture?
Various methods can be employed to separate pure substances from mixtures, including distillation, filtration, and chromatography.
What are some real-world applications of pure substances and mixtures?
Pure substances find applications in pharmaceuticals, electronics, and materials science, while mixtures are essential in everyday products like beverages, cosmetics, and fuels.