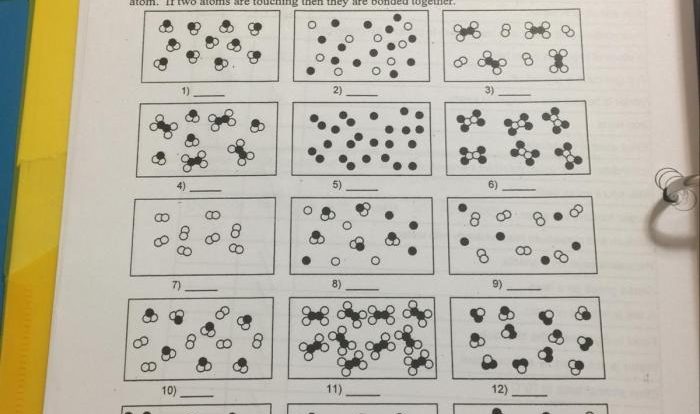
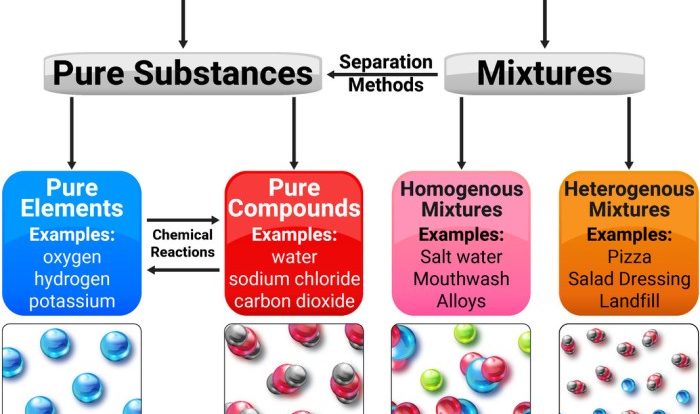
Indicate whether the following balanced equations involve oxidation-reduction. Oxidation-reduction reactions, also known as redox reactions, are chemical reactions that involve the transfer of electrons between atoms or ions. These reactions are characterized by changes in the oxidation states of the reactants and products.
In this article, we will provide a concise definition of oxidation-reduction reactions, explain the concept of electron transfer, and describe the characteristics that distinguish oxidation-reduction reactions from other chemical reactions. We will also analyze several balanced equations to determine whether they involve oxidation-reduction and provide guidance on identifying oxidation and reduction half-reactions within the equations.
Identifying Oxidation-Reduction Reactions
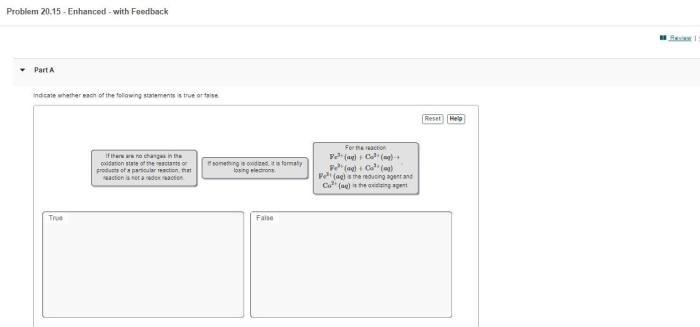
Oxidation-reduction reactions, also known as redox reactions, involve the transfer of electrons between atoms or ions. They are characterized by a change in the oxidation state of the reactants.
Oxidation is the loss of electrons, while reduction is the gain of electrons. In a redox reaction, the species that is oxidized is called the reducing agent, and the species that is reduced is called the oxidizing agent.
Analyzing Balanced Equations
To determine whether a balanced equation involves a redox reaction, we need to identify whether there is a change in the oxidation state of any of the elements involved.
Consider the following balanced equations:
- CH 4+ 2O 2→ CO 2+ 2H 2O
- 2Na + Cl 2→ 2NaCl
- 2Fe + 3Cl 2→ 2FeCl 3
In the first equation, carbon changes from an oxidation state of -4 in CH 4to +4 in CO 2, while oxygen changes from 0 in O 2to -2 in H 2O. This indicates that a redox reaction has occurred.
In the second equation, there is no change in the oxidation state of any of the elements, so this is not a redox reaction.
In the third equation, iron changes from 0 in Fe to +3 in FeCl 3, while chlorine changes from 0 in Cl 2to -1 in FeCl 3. This also indicates that a redox reaction has occurred.
Determining Oxidation States
The oxidation state of an element is a number that represents the hypothetical charge that an atom of the element would have if all of its bonds to other atoms were ionic.
To determine the oxidation state of an element, we can use the following rules:
- The oxidation state of an element in its elemental form is 0.
- The oxidation state of a monatomic ion is equal to the charge of the ion.
- The oxidation state of hydrogen is +1 when bonded to nonmetals and -1 when bonded to metals.
- The oxidation state of oxygen is -2 when bonded to most elements.
- The sum of the oxidation states of all the atoms in a compound is equal to the charge of the compound.
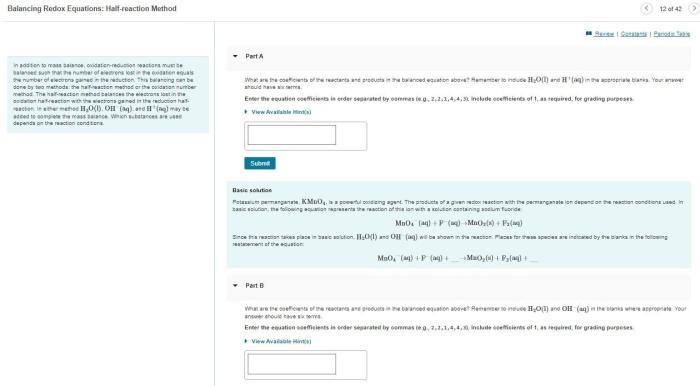
Balancing Oxidation-Reduction Equations: Indicate Whether The Following Balanced Equations Involve Oxidation-reduction

To balance redox equations, we can use the half-reaction method.
In the half-reaction method, we first divide the overall reaction into two half-reactions, one for oxidation and one for reduction.
We then balance each half-reaction in terms of mass and charge.
Finally, we combine the two half-reactions to get the overall balanced equation.
FAQ Corner
What is an oxidation-reduction reaction?
An oxidation-reduction reaction is a chemical reaction that involves the transfer of electrons between atoms or ions.
What is the difference between oxidation and reduction?
Oxidation is the loss of electrons, while reduction is the gain of electrons.
How can I identify oxidation-reduction reactions?
Oxidation-reduction reactions can be identified by changes in the oxidation states of the reactants and products.